Contents
Introduction
Since the 1980s, titanium implants have become a cornerstone of dentistry, largely due to the pioneering work of Dr. Per-Ingvar Brånemark. The earliest implants installed using his technique remain functional today, more than 40 years later. Titanium’s unique ability to fuse directly with bone tissue (osseointegration) creates a stable bond that allows for the complete restoration of a patient’s masticatory function and aesthetics.
Early implants featured smooth, machined surfaces with minimal modification. While osseointegration was often successful with these designs, the failure rate was significantly higher than it is today. For example, retrospective data from early studies show 5-year survival rates for implants supporting fixed prostheses at approximately 75–85%, compared to the 95-98% 5-year survival rate commonly achieved in similar cases today.
Over time, it became evident that textured, rough surfaces and various coatings stimulate osteoblast proliferation, enhance tissue adhesion, and accelerate the osseointegration process. Currently, under favorable conditions, the success rate of integration approaches 100%, and the time required for complete healing has been reduced from 6–9 months to as little as 1.5–3 months, depending on the patient and the implant site.
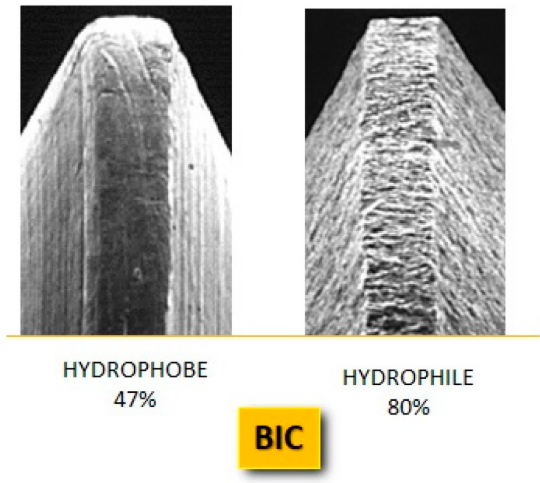
Electron microscopy detail of a smooth (hydrophobe) and a rough (hydrophile) implant surface, with re-wetting percentages. The hydrophilic surface has a higher BIC percentage than the hydrophobic surface. MDPI/ Angelo Michele Inchingolo/ jfb14050287/ April 2023
The surfaces of modern implants are almost always modified. Some manufacturers use texturing (creating a rough or moderately rough surface), while others apply coatings such as hydroxyapatite, calcium phosphate, or even silver and other nanomaterials with antibacterial properties. The coating’s composition, topography, surface chemistry, and application method all influence clinical success. It is especially important to select an implant with properties tailored to the specific clinical situation.
For the practicing clinician, time is a precious resource. Navigating the complexities of different surface coatings—understanding their composition, indications, and benefits—can be challenging in a busy practice. This makes the comprehensive review by Angelo Michele Inchingolo and his colleagues (Surface Coatings of Dental Implants: A Review, MDPI, 2023): which serves as the foundation for this article, particularly valuable. The goal of this article, mirroring that of the original review, is to identify the implant surface characteristics that promote optimal peri-implant tissue healing and ensure long-term clinical success.
Goals and Objectives
The implant surface, with its specific structure and chemical composition, is one of its most critical characteristics. It dictates how quickly bone cells interact with the implant, how well soft tissues heal, and whether the risk of inflammatory complications is minimized. Consequently, surface modification has become a major area of innovation, spanning from sandblasting and acid-etching to complex nanostructures and multifunctional coatings.
The objective of the review conducted by the Italian research team led by Angelo Michele Inchingolo (MDPI, 2023) was to systematize the accumulated knowledge and compare different types of dental implant coatings.
The authors set out to address several specific tasks:
- To collect and classify existing coatings, from classic hydroxyapatite to novel nanostructured and biomimetic materials.
- To evaluate the effect of these coatings on osseointegration, healing rates, and the long-term stability of implants.
- To explore the antibacterial properties of various surface modifications and their role in preventing peri-implantitis.
- To identify the advantages and limitations of different technologies based on both laboratory and clinical data.
- To outline future prospects: determining which coatings are still experimental versus those that have proven their efficacy and are ready for routine clinical use.
In essence, the researchers sought to answer a question pertinent to every practicing dentist:
👉 “What are the key factors to consider when choosing an implant? Which characteristics are clinically significant, and which are primarily marketing features designed to justify a higher price for a standard implant system?”
Review Methods and Design
The authors of the review conducted a systematic search of publications in three major scientific databases—PubMed, Scopus, and Web of Science—to gather comprehensive data on dental implant coatings.
The search was based on a combination of keywords: “dental implants,” “surface coatings,” “osseointegration,” and “antibacterial coatings.” The final selection included articles that were:
- Published in English
- In vivo human studies
- Published in open-access journals
- Described coatings, their properties, and their impact on osseointegration or clinical outcomes
The authors categorized the selected publications based on:
- Coating type (mineral, nanostructured, organic, antibacterial, hybrid);
- Application method (plasma spraying, anodization, laser technologies, etc.);
- Intended effect (accelerated osseointegration, improved soft tissue healing, inflammation prevention);
- Level of evidence (in vitro, in vivo, or clinical data).
Thus, the review’s design can be described as a multi-level comparative analysis, integrating laboratory and preclinical findings with clinical data. This approach provides a holistic view, from fundamental mechanisms to practical implications for clinicians.
Theoretical Basis: Osseointegration and the Implant Surface
When discussing dental implants, the key to success is osseointegration. This term, coined by Albrektsson in 1981, describes the unique ability of titanium to form a direct structural and functional bond with living bone. Essentially, osseointegration is a process where bone cells (osteocytes and osteoblasts) not only adhere to the implant surface but also form a strong, lasting biological connection with it.
How It Works at the Microscopic Level
Following implant placement, a cascade of biological events is initiated:
- Blood Coagulation and Matrix Formation: A protein film immediately forms on the implant surface, creating a scaffold for cellular attachment.
- Inflammatory Response: Immune cells migrate to the surgical site to clear debris and release signaling molecules that stimulate new tissue growth.
- Bone Formation: Osteoblasts begin to produce a bone matrix, which gradually mineralizes.
- Remodeling: Mature bone tissue is formed, becoming tightly integrated with the implant surface.
Why is the Surface So Important?
While a smooth titanium surface can support osseointegration, microscopic irregularities and roughness significantly enhance this process. Cells attach more readily to a textured surface, creating more contact points and accelerating bone matrix growth. Surfaces with different levels of roughness also exhibit distinct hydrophilic and hydrophobic properties.

Fibrin implant wettability. The hydrophobe surface shows poor wettability, unlike the hydrophile one with good wettability. The red arrow indicates the magnitude of liquid permeability on the surface of the implant. MDPI/ Angelo Michele Inchingolo/ jfb14050287/ April 2023
Compared to hydrophobic structures, hydrophilic surfaces facilitate interaction with biological fluids and cells due to their superior surface wettability. This effect, along with the methods used to enhance hydrophilicity, will be discussed in more detail below.
Modern technology has advanced beyond simply creating an irregular titanium surface. Today, implant surfaces can be:
- Micro-rough (e.g., after sandblasting or acid-etching);
- Nanostructured, featuring patterns and channels at the nanometer scale that influence cell behavior;
- Coated with active materials, from calcium phosphates to antibacterial metals like silver.
What Does This Mean in Practice?
- More rapid healing after placement.
- Higher success rates of integration (in modern systems, up to 95–98% or higher).
- Reduced risk of peri-implantitis, especially if the surface has antibacterial properties.
Therefore, the implant surface is not merely “bare metal” but a bioengineered platform that largely determines whether the implant will become a functional part of the body or be rejected. Long-term success depends not only on bone-level integration but also on the formation of a healthy soft-tissue seal. This is where antibacterial surfaces can be beneficial, although they are not without risks.

Schematic image of the implant–bone interface MDPI/ Angelo Michele Inchingolo/ jfb14050287/ April 2023
Classification of Implant Coatings
Main Groups of Coatings and Their Mechanisms
- Mineral coatings (CaP, Hydroxyapatite, etc.)
— Examples: hydroxyapatite (HA), other calcium phosphate coatings.
— Mechanism: Create a bioactive “scaffold” for bone formation; stimulate contact osteogenesis.
— Clinical Effect: Enhances primary osseointegration and accelerates the early healing phase. - Nanostructural and Topographic Modifications (Anodization, TNP, SLA, etc.)
— Examples: Titanium Nanoporous (TNP) surface via anodization, Sandblasted Large-grit Acid-etched (SLA), Dual Acid-Etched (DAE).
— Mechanism: Micro- and nano-roughness increases protein film adhesion and facilitates osteoblast attachment and differentiation.
— Clinical Effect: Increases early implant stability, allowing for earlier loading. Anodization is relatively inexpensive and controllable, creating a porous structure favorable for both bone and soft tissue integration. - Plasma and Thermal Spraying (TPS, etc.)
— Examples: Titanium Plasma Spray (TPS), plasma spraying of hydroxyapatite.
— Mechanism: A layer of desired thickness and roughness is sprayed onto the surface, often to create a highly textured relief.
— Clinical Effect: Provides good primary mechanical fixation; however, the quality of deposition and layer adhesion are critical for long-term success. - Biomimetic and Organic Coatings (Polymers, Collagen, Chitosan)
— Examples: Biopolymers (chitosan), collagen matrices, polyhydroxyalkanoates (PHAs).
— Mechanism: Mimic the extracellular matrix (ECM), improve cell adhesion, and can serve as carriers for growth factors.
— Clinical Effect: Potentially improves soft tissue healing and vascularization; requires controlled biodegradation. - Bioglass and Bioactive Ceramics
— Example: Bioglass 45S5 (a calcium-sodium-silicate composition).
— Mechanism: Releases ions that stimulate osteogenesis and angiogenesis.
— Clinical Effect: Useful for bone regeneration and stimulation; used as standalone coatings or in combination with other materials. - Antibacterial Coatings (Metal Ions, Alloys, Compounds)
— Examples: Copper (Cu), silver (Ag), Ti-5Cu alloys.
— Mechanism: Metal ions exhibit broad-spectrum antibacterial effects, preventing biofilm formation and stability.
— Clinical Effect: Reduces the risk of bacterial colonization and peri-implantitis. However, biocompatibility and the duration of ion release must be carefully evaluated. The review notes the high efficacy of copper and Cu-containing alloys in laboratory studies, as well as their osteogenic potential. - Hybrid and Multifunctional Coatings
— Concept: Combine osteogenic and antimicrobial functions (e.g., CaP + metal ions or a polymer + growth factor).
— Clinical Effect: Potentially achieve an optimal balance between accelerated osseointegration and long-term protection against infection; requires large-scale clinical trials for validation.
Other Important Factors: Composition and Additives
The review emphasizes that adding inorganic bioactive elements (e.g., calcium, phosphorus, fluoride, and graphene oxide) can enhance osteogenesis and angiogenesis. Some elements, such as copper, simultaneously provide an antimicrobial effect and stimulate bone formation. However, different approaches have their own limitations, ranging from corrosion risks to potential cytotoxicity at high ion concentrations.
Surface cleanliness is also critical. Impurities can slow osseointegration or even trigger rejection. Manufacturers address this in various ways. For instance, XGATE Dental uses a multi-step process marketed as Pure & Porous (P& P). The goal is to achieve a high-quality surface structure similar to SLA but without its drawbacks. The SLA process involves sandblasting with aluminum oxide particles, followed by acid etching to dissolve any abrasive particles embedded in the surface. An alternative technique, RBM (Resorbable Blast Media), uses hydroxyapatite particles, which are a desirable component. However, hydroxyapatite is much softer and, as an abrasive, cannot create the same complex peak-and-valley structure as aluminum oxide.
XGATE Dental’s solution uses non-aggressive abrasive particles that still produce a high-quality surface. The scanning electron microscope (SEM) image below (5000x magnification) shows the result.

The surface microstructure of SLA and P& P samples reveals a well-organized, two-tiered topography—cavities 10-30 microns wide, lined with craters 1-3 microns in diameter. This microstructure is internationally recognized as optimal for bone cell proliferation and osseointegration.
The surface microstructure of SLA and P& P samples reveals a well-organized, two-tiered topography—cavities 10-30 microns wide, lined with craters 1-3 microns in diameter. This microstructure is internationally recognized as optimal for bone cell proliferation and osseointegration.
In contrast, the surface of RBM implants has a chaotic topography without a regular microstructural pattern.
Furthermore, the surface cleanliness of XGATE Dental implants regarding aluminum derivatives is comparable to that of implants processed using RBM technology.
Brief Comparison Table (Type → Mechanism → Clinical Benefits/Limitations)
| Type of coating | Example(s) | The main mechanism | Clinical benefit | Main limitations |
|---|---|---|---|---|
| Minerals (CaP, HA) | Hydroxyapatite, CaP | Biological “scaffold” for bone formation | Accelerates contact osteogenesis; improves early integration | Variable deposition quality; risk of layer delamination |
| Nanostructured / topography | Anodizing (TNP), SLA, DAE | Micro/nano roughness → improved cell adhesion | Rapid primary stability; better soft tissue integration | Requires precise process control; potential for corrosion if passivation is poor |
| Plasma spraying (TPS) | TPS, plasma HA | Thick, rough layer for mechanical fixation | Excellent initial fixation | Spraying quality is critical; risk of coating delamination |
| Biomimetic/polymeric | Chitosan, collagen, PHAs | ECM mimicry, growth factor carrier | Improves soft tissue healing | Biodegradation; limited long-term data |
| Bioglass / bioactive ceramics | Bioglass 45S5 | Ion release → osteogenesis/angiogenesis | Supports regeneration of bone defects | Brittleness; integration with metal requires optimization |
| Antibacterial (metals) | Cu, Ag, Ti-5Cu | Ions suppress biofilm and bacteria | Reduces risk of colonization/peri-implantitis | Risk of cytotoxicity at high concentrations; corrosion |
| Hybrid coatings | CaP + Ag/Cu; polymer + BMP | Combination of osteoinduction + protection | Potentially the best combination of effects | Manufacturing complexity; requires more clinical evidence |
(All key points in the table are based on systematic data from the MDPI 2023 survey.)
Visual Diagram (Mermaid): Effects of Coating Groups
Coating Application Methods
Modern technologies for modifying dental implant surfaces are extremely diverse. This review highlights several key approaches that alter the titanium’s topography or add functional layers.
1. Plasma Spraying
One of the oldest and most common methods, it involves atomizing material particles (e.g., hydroxyapatite) in a plasma jet and depositing them onto the titanium surface. The result is a layer with distinct roughness and a specific thickness.
- Advantages: Ability to apply thick coatings, high processing speed, good primary mechanical fixation.
- Disadvantages: Risk of layer delamination, structural heterogeneity, quality is highly dependent on the technology.
Clinical studies show good osseointegration with plasma-sprayed HA coatings; however, coating detachment can lead to inflammation and implant loss.
2. Anodization and Nanoporous Structures
An electrolytic process that creates nanopores of varying diameters on the titanium surface.
- Advantages: Relatively simple process, allows control over pore size and distribution, as well as the thickness of the oxide layer.
- Mechanism: Nanopores increase the surface area and improve osteoblast adhesion. These structures can also serve as reservoirs for bioactive molecules.
3. Laser Processing
Lasers can create micro- and nanostructures with high precision.
- Advantages: High reproducibility and accuracy, potential for localized modification.
- Disadvantages: High equipment cost, requires strict parameter control.
Laser processing is often combined with other methods, such as acid-etching.
4. Biomimetic Deposition
This method mimics the natural formation of bone tissue. The implant is placed in a solution saturated with calcium and phosphate ions, leading to the precipitation of hydroxyapatite on its surface.
- Advantages: High biocompatibility of the coating, chemical similarity to bone tissue.
- Disadvantages: A lengthy process, and the resulting layer has relatively low mechanical strength.
5. Ion Implantation and Chemical Etching
- Ion Implantation: Allows atoms of other elements (e.g., silver or copper) to be embedded into the titanium surface, imparting antimicrobial properties.
- Acid Etching: Often combined with sandblasting, this method creates a micro- and nano-topography that increases the surface area for cell contact.
Hydrophilic and Hydrophobic Surfaces
As mentioned earlier, surface wettability plays a critical role in osseointegration.
- Hydrophilic surfaces promote rapid protein adsorption, facilitating osteoblast attachment and bone matrix formation. These surfaces accelerate the initial stages of healing and can reduce the time to complete osseointegration.
- Hydrophobic surfaces, in contrast, are less effective at attracting proteins and cells, which can slow the integration process and reduce the predictability of the outcome.
Modern processing methods aim to create superhydrophilic surfaces. Examples include:
- SLA (sandblasting + acid treatment) combined with subsequent plasma treatment.
- Modifications that keep the surface in a sterile, moist environment until implantation.
The authors note that studies consistently show implants with hydrophilic surfaces exhibit higher rates of Bone-to-Implant Contact (BIC) in the first weeks post-placement compared to traditional hydrophobic implants.
A hydrophilic surface ensures immediate contact with the blood clot, which is crucial for initiating osseointegration. Some surfaces are so hydrophilic that as soon as the implant touches blood, it wicks across the entire surface (see illustration). Rough surfaces further enhance clot retention.

Good implant wettability: As soon as the implant is inserted into the bone, there is immediate blood–fixture contact. Blood is attracted to the implant surface. MDPI/ Angelo Michele Inchingolo/ jfb14050287/ April 2023
The implant surface after creating a microrelief has much better retraction of fibrin fibers and blood clotting, see the illustration below.

Summary diagram of implant surface treatments. The diagram shows how the hydrophilic property of the implant surfaces (treated and hybrid) lends itself better than the hydrophobic surfaces (machined and smooth) to further treatments to improve their general characteristics. MDPI/ Angelo Michele Inchingolo/ jfb14050287/ April 2023
Comparative Analysis of Coatings: Clinical and Experimental Data
The systematic review encompassed a wide range of studies, from in vitro experiments to clinical observations. The results were categorized to evaluate:
- Level of osseointegration (Bone-to-Implant Contact, BIC),
- Implant stability (often assessed using Resonance Frequency Analysis, RFA),
- Antibacterial properties,
- Clinical survival rates of implants.
1. Mineral Coatings (CaP, Hydroxyapatite)
- In vivo studies showed a significant increase in BIC compared to control titanium surfaces.
- In clinical trials, HA coatings have demonstrated high survival rates (often >95% over a 5-year period), with the caveat that coating quality is critical. Cases of delamination have led to implant loss.
2. Nanostructured Surfaces (Anodization, SLA, DAE)
- Anodized surfaces demonstrate accelerated bone contact formation and high stability values in the early stages (4–8 weeks).
- SLA and DAE surfaces provide consistently high BIC and are considered a “gold standard” in clinical practice.
- The review confirms that SLA and anodization show equally high implant survival rates of around 97–99% in long-term follow-up.
3. Plasma Spraying (TPS, HA-TPS)
- TPS increases mechanical adhesion, but coating durability can be a concern.
- Plasma-applied HA demonstrates improved BIC in the early stages, but some long-term studies have documented an increased risk of complications if the coating fails.
4. Biomimetic and Organic Coatings
- Chitosan and collagen layers improve cellular adhesion in vitro.
- Clinical data are still limited; efficacy is primarily supported by preclinical models.
5. Bioglass and Ceramics
- Bioglass 45S5 stimulates osteogenesis and angiogenesis; in vivo data show a significant increase in BIC.
- Clinical trials are limited, but preliminary results indicate good potential for use in complex bone defects.
6. Antibacterial Coatings (Ag, Cu and their alloys)
- In vitro studies showed marked suppression of biofilms and bacterial growth (e.g., Staphylococcus aureus, Porphyromonas gingivalis).
- Cu coatings and Ti-5Cu alloys have also been shown to stimulate osteogenesis.
- Sufficient clinical data are not yet available, but this is an active area of development.
Comparison table
| Type of coating | Evidence (in vitro / in vivo / clinical) | BIC / osseointegration | Clinical survival rate | Limitations |
|---|---|---|---|---|
| Minerals (CaP, HA) | in vivo + clinical | ↑ Significant BIC increase | >95% (5 years) with high quality | Risk of delamination |
| SLA / DAE / anodization | all levels | High BIC | 97–99% long-term | Requires precise process control |
| Plasma spraying (TPS, HA-TPS) | in vivo + clinical | ↑ BIC in early stages | High survival, but dependent on coating | Potential for layer separation |
| Biomimetics (chitosan, collagen) | in vitro, limited in vivo | Improves cell adhesion | Limited clinical data | No long-term data |
| Bioglass / ceramics | in vivo, limited clinical | Stimulates osteogenesis | Potentially high | Brittleness, limited research |
| Antibacterial (Ag, Cu) | in vitro, limited in vivo | Combines osteogenesis and antimicrobial effects | Limited clinical data | Cytotoxicity, corrosion |
Practical Implications for Clinicians and Review Conclusions
The findings of this systematic review (Inchingolo A. M. et al., MDPI, 2023) clearly demonstrate that the evolution of dental implant coatings has transformed clinical practice. Whereas successful osseointegration in the 1980s was less predictable and associated with a significant failure rate, today’s diverse surface modification methods provide clinicians with tools to increase success rates to 97–99% and beyond.
What does this mean for a practicing clinician?
- SLA, DAE, anodized surfaces
- Optimal for general practice, as they provide reliable and rapid osseointegration.
- Long-term implant survival rates are consistently in the 97–99% range.
- These technologies can be considered the “gold standard” for most clinical cases.
- Mineral coatings (CaP, hydroxyapatite)
- Accelerate integration, especially in cases of compromised bone quality.
- Suitable for patients with osteopenia or when a shorter healing time before prosthetic loading is desired.
- Require strict quality control, as coating delamination is associated with a risk of implant failure.
- Antibacterial coatings (Ag, Cu)
- Promising for patients at high risk of inflammatory complications (e.g., with a history of peri-implantitis).
- Lack a sufficient clinical evidence base and should currently be considered experimental solutions.
- Bioglass and ceramics
- Show great potential for complex reconstructions and bone defects.
- Can stimulate not only osteogenesis but also angiogenesis, which is important when working in areas with a limited blood supply.
- Limitation: Insufficient clinical data for widespread routine use.
- Biomimetic coatings (chitosan, collagen)
- Show good results in cell adhesion and soft tissue healing at the preclinical level.
- Promising for aesthetic zones where soft tissue profile management is critical.
- However, convincing long-term clinical data is currently lacking.
Key Findings of the Review
- Implant surface modification is a critical factor for successful osseointegration and treatment longevity.
- Surface hydrophilicity plays a key role: hydrophilic coatings accelerate the attachment of proteins and cells, reducing integration time.
- Universal technologies (SLA, anodization) remain the most predictable and clinically proven options to date.
- New coating types (antibacterial coatings, bioglass, biomimetics) have great potential but require further clinical investigation.
Research limitations
- Much of the data was obtained from in vitro and in vivo models; the clinical evidence for many coatings (especially innovative ones) remains limited.
- There is a lack of standardized comparison protocols; different studies use varying assessment timelines and metrics (BIC, RFA, survival rates).
- A publication bias may be present, as studies with positive results are more likely to be published than those with neutral or negative findings.
Disclaimer: Any medical or scientific information provided in connection with the content presented here makes no claim to completeness and the topicality, accuracy and balance of such information provided is not guaranteed. The information provided by XGATE Dental Group GmbH does not constitute medical advice or recommendation and is in no way a substitute for professional advice from a physician, dentist or other healthcare professional and must not be used as a basis for diagnosis or for selecting, starting, changing or stopping medical treatment.
Physicians, dentists and other healthcare professionals are solely responsible for the individual medical assessment of each case and for their medical decisions, selection and application of diagnostic methods, medical protocols, treatments and products.
XGATE Dental Group GmbH does not accept any liability for any inconvenience or damage resulting from the use of the content and information presented here. Products or treatments shown may not be available in all countries and different information may apply in different countries. For country-specific information please refer to our customer service or a distributor or partner of XGATE Dental Group GmbH in your region.