A clinical case presented by Dr. Michael Carmy from Rialto, California, USA.

This case study by Dr. Carmy demonstrates a comprehensive approach to full maxillary rehabilitation and partial mandibular restoration using XGATE Dental V-Type multi-unit abutments and implants from multiple systems. The uniqueness of this clinical work lies in the combination of immediate implant placement with the root shield technique — which allowed for maximum bone tissue preservation—and a staged prosthetic approach using a temporary PMMA prosthesis to ensure stable esthetics and comfort throughout the treatment.
Particular attention was paid to optimizing load distribution through the use of low-profile multi-unit abutments and a segmented design for the final zirconia bridges. This approach minimizes the risk of overload and increases the long-term durability of the restoration.
This case exemplifies modern rehabilitation, where XGATE Dental technologies help achieve stable biomechanical outcomes and natural esthetics, even in clinically challenging conditions.
Patient Summary
The patient is a relatively young man in recovery from drug addiction. At the time of his initial consultation, he had been living a healthy lifestyle for three years. Restoring his smile was a high priority, as it is crucial not only for esthetics but also for self-confidence, clear speech, and the ability to maintain a nutritious diet without limitations.
Long-term psychoactive substance use had caused severe xerostomia, contributing to the rapid development of dental caries and decay. The teeth in the upper jaw were non-restorable, and many were already missing. The condition of the lower jaw was slightly better: the anterior teeth were in satisfactory condition, but the posterior teeth were severely decayed and required extraction.
The presented images show the patient’s initial dental condition.







Treatment Plan and Its Adjustment During Execution
The initial plan was to rehabilitate the upper jaw using an All-on-4 protocol with an FP3 Tx prosthesis, which replaces not only the tooth crowns but also the lost gingival (soft) tissue. This approach would have required a significant reduction of the alveolar ridge to accommodate the full-arch prosthesis.
However, after a detailed assessment of the bone volume, height, and density, the decision was made to adjust the treatment plan in favor of a more conservative, bone-preserving strategy.
Stage 1
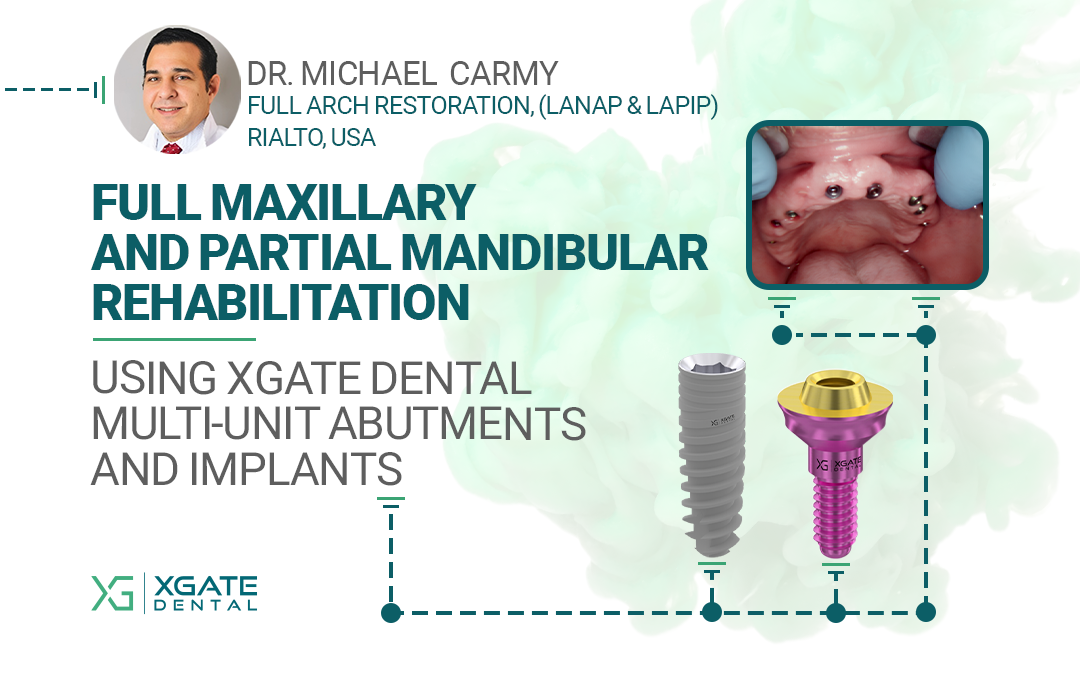
The implants were placed immediately following tooth extraction. They were positioned subcrestally — 2–3 mm below the bone crest — to allow for anticipated bone remodeling. A total of eight implants were placed in the maxilla and four in the mandible.
Placing the implants into the fresh extraction sockets allowed for optimal positioning for the future prosthetic restoration. The extractions were performed using the root shield technique, which is aimed at maintaining the thickness and height of the buccal and palatal bone walls. This technique, traditionally used for “conservation” even in case of delayed implantation, helps to minimize resorption and preserve the natural morphology of the alveolar ridge.




Implant Placement Diagram
| Tooth Position (FDI) | Implant System | Size (Ø x L) and Purpose |
|---|---|---|
| #11, #12 | XGATE Dental | 3.75 x 15.2 mm (Anterior Maxilla) |
| #34 | XGATE Dental | 4.2 x 10 mm (Mandibular Premolar) |
| #16, #26 | DSI Implants | 5.0 x 10 mm (Molar Region) |
| #13, #14, #23 | DSI Implants | 3.75 x 15.2 mm (Anterior/Premolar Region) |
| #36, #45, #47 | DSI Implants | 4.2 x 10 mm or 5.0 x 10 mm (Mandibular Region) |
| Total: 12 Implants | 3 XGATE, 9 DSI (Correction: 8 DSI) | Optimal Prosthetic Support |


Implant dimensions were selected based on the patient’s anatomy and the anticipated functional load in each area:
- Molar region: 5.0 x 10 mm
- Anterior region: 3.75 x 15.2 mm
- Mandibular premolar region: 4.2 x 10 mm
For the first two weeks post-surgery, the patient was instructed to follow a soft-liquid diet to promote optimal tissue healing and minimize swelling. Once the mucosa had stabilized and initial healing was complete, the first temporary prosthesis could be delivered.
Stage 2
Two weeks after implant placement, a temporary removable prosthesis was delivered. During this phase, the implants remained non-loaded to allow for undisturbed osseointegration. The prosthesis was carefully designed to support the gingival contours and preserve the interdental papillae, a key factor for achieving optimal esthetics in the final restoration.








The planned final maxillary restoration will consist of several independent zirconia bridges. Separating the posterior and anterior segments in this way creates shorter lever arms, which reduces torque and stress on the implants, preventing loosening and overload.
Currently, the patient is wearing the provisional PMMA prosthesis. He is attending regular professional hygiene appointments and adhering to a strict home care regimen. This transitional period allows for the evaluation of the function and esthetics of the PMMA prosthesis, so that any necessary adjustments can be made before fabricating the final restoration, ensuring an optimal outcome.
The patient’s appearance at this stage demonstrates a natural and harmonious result.

XGATE Dental Products Used in Case Study
This clinical case demonstrates a full maxillary rehabilitation and partial mandibular restoration using XGATE X3 Internal Hex implants and V-Type Multi-Unit Abutments.
Download Resources
Get the complete case study and explore our product catalog
Case Study • PDF • 2.2 MB
📥 Download Case StudyProduct Catalog • Online
🔍 View Product CatalogWe hope you found this clinical case interesting. If you have any questions about the characteristics and delivery of XGATE Dental products, please contact us in any convenient way.
XGATE Dental Group GmbH
Falkensteiner Straße 77, 60322
Frankfurt am Main
Germany
E-mail: [email protected]
350 W Passaic
St Rochelle Park, NJ 07662
United States
Disclaimer: Any medical or scientific information provided in connection with the content presented here makes no claim to completeness and the topicality, accuracy and balance of such information provided is not guaranteed. The information provided by XGATE Dental Group GmbH does not constitute medical advice or recommendation and is in no way a substitute for professional advice from a physician, dentist or other healthcare professional and must not be used as a basis for diagnosis or for selecting, starting, changing or stopping medical treatment.
Physicians, dentists and other healthcare professionals are solely responsible for the individual medical assessment of each case and for their medical decisions, selection and application of diagnostic methods, medical protocols, treatments and products.
XGATE Dental Group GmbH does not accept any liability for any inconvenience or damage resulting from the use of the content and information presented here. Products or treatments shown may not be available in all countries and different information may apply in different countries. For country-specific information please refer to our customer service or a distributor or partner of XGATE Dental Group GmbH in your region.