The key feature of this case is the demonstration of the reliability of XGATE multi-unit abutments, even during the temporary rehabilitation phase. In this clinical situation, it was not possible to immediately use all four implants to support the prosthesis. However, the temporary structure, retained by only three screws, functioned effectively for the four months required to achieve osseointegration.
By the time the final prosthesis was delivered, the situation had been successfully resolved. Secondary stability was achieved through the natural biological process, and the milled bar fit all four multi-unit abutments precisely and passively.
This case is a clinical confirmation that, despite unforeseen circumstances, having a margin of safety and alternative solutions is extremely important.

Today we present another clinical case from
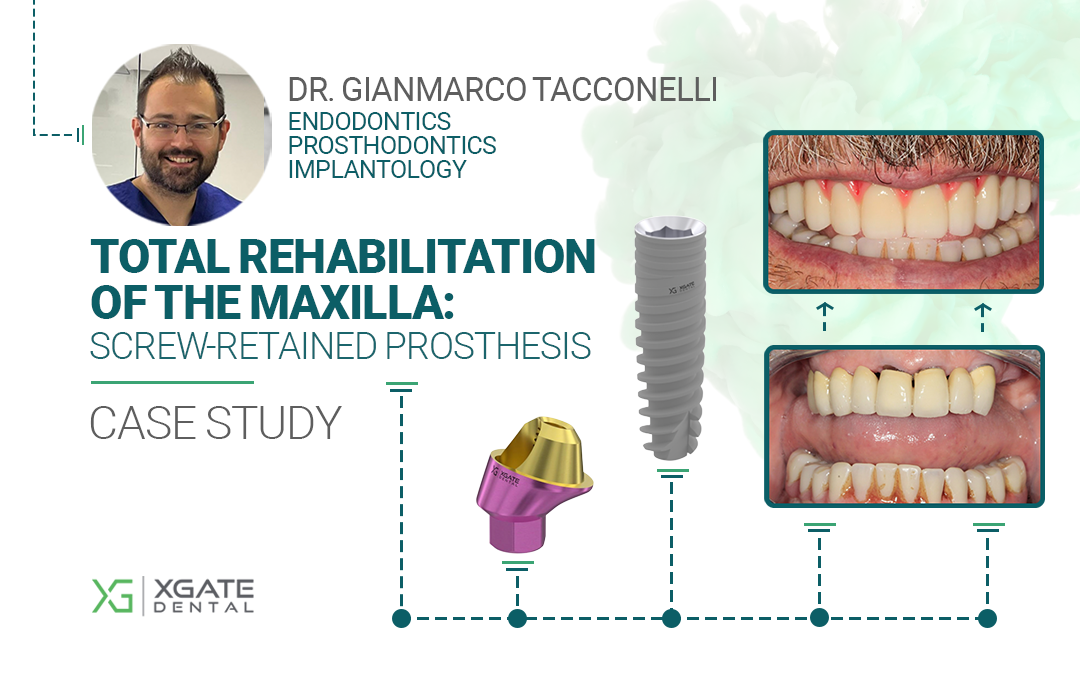
Dr. Gianmarco Tacconelli of Pescara, Italy.
With over 11 years of clinical experience, Dr. Tacconelli specializes in maxillary implantology and rehabilitation, with advanced skills in zygomatic and pterygoid implants, as well as bone grafting for severe atrophy.
Main areas of expertise: Endodontics, Prosthodontics, and Implantology.
Patient Overview
A 73-year-old man in good general health presented to the clinic with a damaged upper overdenture, which was supported by the roots of his natural teeth. The initial condition is clearly visible in the photographs and CBCT scans. After a clinical and radiographic evaluation, the remaining roots were deemed non-restorable and unsuitable for supporting a new prosthesis. The patient’s bar-retained lower denture was assessed as being in good condition and did not require intervention.



Treatment Plan and Procedure
After assessing the clinical and radiographic condition of the maxilla, as well as the quantity and quality of bone available for implant-supported prosthetic rehabilitation, the following treatment plan was established:
- Extraction of the remaining non-restorable tooth roots.
- Alveoloplasty in the anterior region.
- Placement of four implants for a screw-retained, Toronto Bridge-style prosthesis (a prosthesis with an artificial gingiva).
The extractions of the roots were carried out without complications.

The next step was to perform the alveoloplasty to create sufficient vertical space for the framework and prosthetic components.

Next, the implants were placed. Based on the CBCT data and the patient’s anatomy, the placement of the following XGATE Dental implants and multi-unit abutments was planned:
| Tooth Position | Implant (Ø x L) | Abutment |
|---|---|---|
| 15 | 3.75 x 13 mm | D-type 30° 1mm |
| 25 | 3.75 x 13 mm | D-type 30° 2mm |
| 12 | 3.75 x 11.5 mm | D-type 17° 1mm |
| 22 | 3.75 x 11.5 mm | D-type 17° 1mm |


XGATE Dental Implant X3 Internal Hex


XGATE Dental MUA D-Type 30°

XGATE Dental MUA D-Type 17°

Next, transfers were placed to take impressions.

Occlusal registration and the vertical dimension of occlusion were recorded using a wax rim.

During this process, difficulties arose with seating the MUA on the implant in the #25 position. Firstly, the screw access channel had an unfavorable trajectory relative to the occlusal plane. Secondly, when attempting to tighten the 30° MUA, the implant showed inadequate primary stability, with a final torque value of only 30-35 Ncm.
It was decided not to immediately load this implant and to wait for secondary stability to be achieved. Only then would the MUA be repositioned to ensure the screw access channel was in the optimal location. Therefore, the MUA was removed and replaced with a healing cap.
A temporary acrylic resin prosthesis, featuring stock teeth and an internal metal reinforcement, was fabricated and secured to the three stable implants. The patient was dismissed and scheduled for a follow-up in four months to allow for complete osseointegration.


After four months, the temporary prosthesis was removed. The XGATE 30° MUA was successfully placed on the implant in the #25 position with the correct orientation. A final impression was then taken using MUA-level impression copings to create a master cast.
Crucially, the new position of the MUA on the #25 implant no longer presented problems: the screw access channel was correctly oriented relative to the occlusal plane, as originally planned.








A prosthetic try-in was performed to assess the occlusion and desired tooth morphology. For additional verification, the try-in was compared with a digital scan of the functional temporary prosthesis. This step preceded another try-in of the tooth setup.


After confirming the vertical dimension of occlusion, a resin verification jig was fabricated to confirm the accuracy of the master cast and to radiographically verify the passive fit of the milled titanium bar.


Finally, the occlusion was checked and the final prosthesis was delivered. The prosthesis consists of a milled PMMA framework with a pink gingiva fabricated by layering composite resin.




The patient’s aesthetics and chewing function were fully restored.
The patient was highly satisfied with the final result.
XGATE Dental Products Used in Case
This clinical case by Dr. Gianmarco Tacconelli demonstrates a full-arch maxillary rehabilitation. The procedure utilized XGATE X3 Internal Hex RP implants and D-type multi-unit abutments. All products used in this case are listed under the 'Maxilla' filter.
Download Resources
Get the complete case study and explore our product catalog
Case Study • PDF • 5.8 MB
📥 Download Case StudyProduct Catalog • Online
🔍 View Product CatalogWe hope you found this clinical case interesting. If you have any questions about the characteristics and delivery of XGATE Dental products, please contact us in any convenient way.
XGATE Dental Group GmbH
Falkensteiner Straße 77, 60322
Frankfurt am Main
Germany
E-mail: [email protected]
350 W Passaic
St Rochelle Park, NJ 07662
United States
Disclaimer: Any medical or scientific information provided in connection with the content presented here makes no claim to completeness and the topicality, accuracy and balance of such information provided is not guaranteed. The information provided by XGATE Dental Group GmbH does not constitute medical advice or recommendation and is in no way a substitute for professional advice from a physician, dentist or other healthcare professional and must not be used as a basis for diagnosis or for selecting, starting, changing or stopping medical treatment.
Physicians, dentists and other healthcare professionals are solely responsible for the individual medical assessment of each case and for their medical decisions, selection and application of diagnostic methods, medical protocols, treatments and products.
XGATE Dental Group GmbH does not accept any liability for any inconvenience or damage resulting from the use of the content and information presented here. Products or treatments shown may not be available in all countries and different information may apply in different countries. For country-specific information please refer to our customer service or a distributor or partner of XGATE Dental Group GmbH in your region.